Splice Serum Download Keeps Freezing
Posted By admin On 10.12.20- Splice Serum Download Keeps Freezing Free
- Splice Serum Download Keeps Freezing On Windows 10
- Xfer Serum Download
- Splice Serum Download Keeps Freezing Windows 10
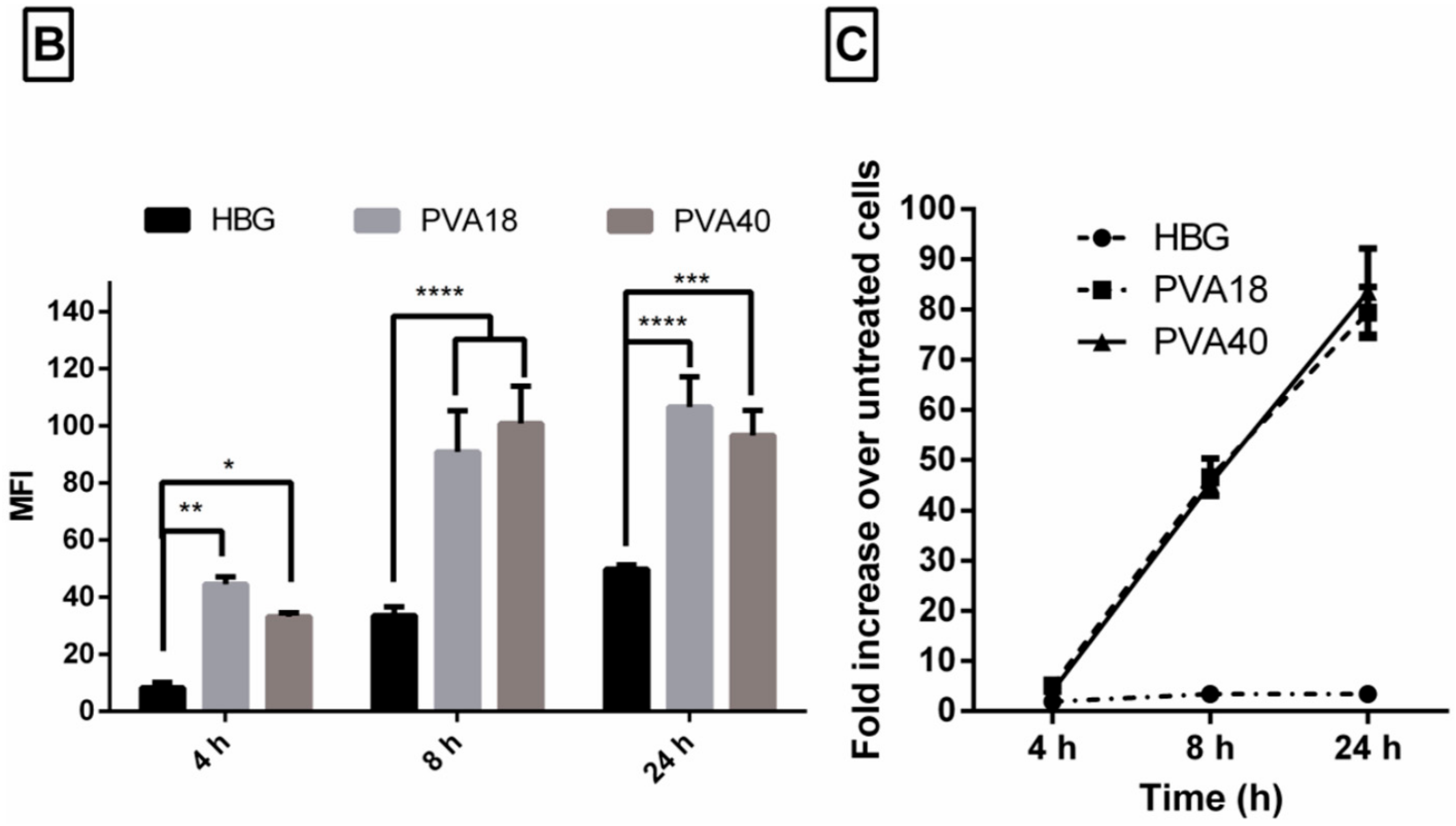
Hi everyone, I am currently on day two of my 'three day trial' of the Splice rent to own program of Serum, but I haven't even gotten to experiment around with it because it is asking for a serial number. Install the Splice desktop app to connect your DAW to the cloud. Back up your work, get projects from the community, and download samples. Under a defined set of conditions. When using serum-free media in cell culture, it is important to cryopreserve cells also in a medium free of serum. The novel cell freezing medium that has been developed by Biological Industries contains no serum, but rather methylcellulose and DMSO. After freezing and thawing, a. Feb 24, 2017 Hi everyone, I am currently on day two of my 'three day trial' of the Splice rent to own program of Serum, but I haven't even gotten to experiment around with it because it is asking for a serial number. Kickstart your next project with samples from Audentity Records - Jersey Club by Audentity Records. Browse, preview and download all 204 samples & loops, or download only the sounds you want. Start with a 14-day free trial, then just $7.99/month.
Abstract
Serum is often frozen and banked for analysis at a later date. This study assessed the stability of 17 analytes in rat serum during refrigeration at 4 °C and extended storage at −20 °C (frost-free and nonfrost-free freezers) and −70 °C. Samples were analyzed by using an automated dry-slide chemistry analyzer at time 0 and then stored as aliquots for analysis at time points including day 7, 30, 90, and 360. After 7 d of refrigeration, only creatine kinase activity had varied by more than 10% of the starting value. Freezing at −70 °C was clearly superior to −20 °C where changes were observed in CO2 as early as day 30 and alanine aminotransferase as early as day 90. Samples stored in frost-free and nonfrost-free −20 °C freezers did not differ significantly through day 90. Factors such as storage time and temperature should be considered when designing any retrospective study.
Experimental design frequently necessitates the use of frozen samples for retrospective studies. The planning for multiple experimental time points often results in samples that will be analyzed together at a later date and thus subjected to different periods of storage before analysis. Acquisition of samples after hours or on weekends often results in a few days of refrigeration before analysis. In addition, many investigators may not have access to −70 °C storage or may use frost-free −20 °C freezers without being aware that doing so may affect their experimental results.
Studies of human blood samples, including stability analyses for refrigeration and the effects of freeze–thawing,-, have resulted in guidelines for storage. Surprisingly, data from such reports in veterinary medicine are rather scarce, with only a few reports on the effects of storage on canine and avian samples., Notably, studies in the veterinary literature indicate that stability differs among species, including humans. The present study examined the stability of 17 biochemical analytes in rat serum after refrigeration and freezing at 2 temperatures and at multiple time points.
Materials and Methods
Animals and housing.
All animals were maintained in accordance with the temperature and humidity recommendations of the Guide for the Care and Use of Laboratory Animals at the facilities of the University of Miami, which are AAALAC-accredited.7 All experimental procedures were approved by the university's Animal care and use committee. As part of the university's rodent health program, sentinel rats are maintained on dirty bedding and screened quarterly for the following agents: Sendai virus, rat coronavirus (sialodacryoadenitis) virus, pneumonia virus of mice, Kilham rat (H1) virus, Mycoplasma pulmonis, rat parvovirus, rat minute virus, and parasitic infections. Once a year, the panel is extended to include the following agents: Thieler murine encephalomyelitis virus, Encephalitozoon cuniculi, mouse adenovirus, reovirus, hantavirus, and cilia-associated respiratory bacillus. All results from the sentinel rats were negative during the course of this investigation.
For this study, Sprague–Dawley rats were obtained from a commercial vendor (Harlan, Indianapolis, IN) and were kept conventionally housed in groups of 2 animals per cage using nonautoclaved bedding (Aspen, Harlan Teklad, Madison WI) and microisolation tops. Rats were given ad libitum access to rodent chow (Lab Diet 5001, PMI International, Richmond, IN) and municipal water by bottle.
Sample collection, storage, and analysis.
Rats were euthanized by CO2 overdose, and blood was collected by cardiac puncture and placed in clot tubes (Terumo Capiject, VWR, West Chester, PA). The blood was allowed to clot for 30 min and the serum was separated by centrifugation. All samples were free of hemolysis and lipemia.
Each study sample was a unique sample pooled from 2 individual animals. A total of 16 rats were bled on a single day for the first study to create 8 serum samples which were analyzed at all time points for the refrigeration, −20 °C, and −70 °C comparison. A total of 20 rats were bled on a single day for the second study to create 10 serum samples that were analyzed in the study comparing −20 °C freezers (frost-free versus nonfrost-free). The serum was analyzed for the day 0 time point and then aliquoted in freezer appropriate O-ring enclosure freezer tubes for storage at either 4 °C (range, 2 °C to 4 °C), −20 °C nonfrost-free (range, −18 °C to −22 °C), −20 °C frost-free (range, −16 °C to −25 °C) or −70 °C (range, −72 °C to −76 °C). The −20 °C freezers were a frost-free freezer that goes through a preprogrammed warm-up cycle to prevent the buildup of frost and a −20 °C freezer that does not have this option and maintains a stable temperature. Samples were analyzed on days 7, 30, 90, and 360. The freezers and refrigerator were monitored for temperature through the use of thermometers and high temperature alarms. All equipment is on automatic emergency power back up.
All chemistry analyses were conducted by using a dry-slide chemistry analyzer (Vitros 250, Ortho, Rochester, NY). Quality assurance controls representing high and low values were run on the analyzer throughout the study. The following analyses were performed: glucose, BUN, sodium, potassium, chloride, total CO2, amylase, lipase, calcium, phosphorus, total protein, albumin, aspartate aminotransferase, alanine aminotransferase, lactate dehydrogenase, creatine kinase, and alkaline phosphatase.
Statistical analysis.
The mean and standard error of samples was calculated, and percentage differences from the starting value were reported. Differences greater than 10% were not only statistically significant (by using paired t test methodology) and represented changes greater than 2 standard deviation of the starting values but also represented the minimal difference that was considered to have clinical significance in the interpretation of the biochemical analysis. All analyses were conducted using GraphPad Prism 4 software (La Jolla, CA).
Results
After samples had been refrigerated for 7 d, several analytes showed slight changes: CO2 decreased by 9.4%, creatine kinase activity by 11.1%, and lactate dehydrogenase by 8.0% (P < 0.05) (Table 1). Significant changes were not detected in samples frozen at −20 °C (frost-free) and −70 °C through day 7. However, creatine kinase activity dropped by 59% and alanine aminotransferase activity decreased by 54% by day 360 in the frost-free freezer (P < 0.05). Other changes (P < 0.05) occurred in CO2, lipase, calcium, and alkaline phosphatase. In the samples stored at −70 °C, none of the chemistry markers had changed by more than 10% by day 360.
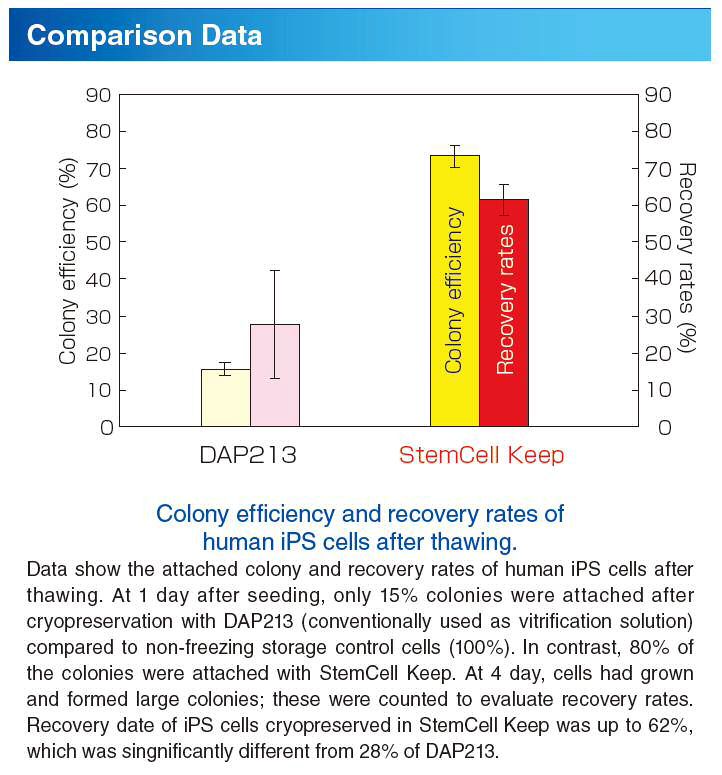
Table 1.
Effects of prolonged refrigeration and freezing on biochemical analytes in rat serum
| Mean ± SE, day 0 | Mean (n = 9 or 10) percentage change from day 0 value | |||
| Analyte | Refrigeration, day 7 | Frost-free −20°C freezer, day 360 | Freezing −70°C, day 360 | |
| Glucose, mg/dL | 153.3 ± 7.0 | 1.6 | (5.8) | 1.3 |
| Blood urea nitrogen, mg/dL | 21.25 ± 0.67 | 0.2 | 6.5 | (0.3) |
| Sodium, mmol/L | 145.8 ± 0.5 | (1.0) | 1.3 | 1.1 |
| Potassium, mmol/L | 5.61 ± 0.07 | (0.7) | (1.2) | 1.2 |
| Chloride, mmol/L | 97.8 ± 0.5 | (0.2) | 3.3 | 2.4 |
| Carbon dioxide, mmol/L | 32.5 ± 0.07 | (9.4)a | (31.1)a | 4.3 |
| Amylase, U/L | 1243.0 ± 37.6 | 1.4 | 0.9 | (0.6) |
| Lipase, U/L | 91.5 ± 2.7 | (0.9) | (19.1)a | 4.4 |
| Calcium, mg/dL | 11.03 ± 0.08 | 0.8 | 17.0a | 3.0 |
| Phosphorus, mg/dL | 8.64 ± 0.15 | (0.5) | 6.8 | 1.4 |
| Total protein, g/dL | 6.35 ± 0.04 | (4.1) | (2.4) | 0.5 |
| Albumin, g/dL | 3.70 ± 0.04 | (2.7) | 6.8 | (1.9) |
| Aspartate transaminase, U/L | 111.9 ± 3.5 | (3.8) | (7.1) | 8.8 |
| Alanine transaminase, U/L | 92.6 ± 1.6 | 0.4 | (54.0)a | (3.6) |
| Lactate dehydrogenase, U/L | 887.0 ± 54.2 | (8.0)a | 2.8 | 3.3 |
| Creatine kinase, U/L | 178.1 ± 19.2 | (11.1)a | (59.3)a | (2.7) |
| Alkaline phosphatase, U/L | 208.9 ± 13.2 | (2.5) | (22.9)a | 5.7 |
Values in parentheses indicate decreases from day 0 value.
In a second study, samples were stored in frost-free and nonfrost-free freezers at −20 °C and analyzed on days 30 and 90 (Table 2). Samples in both freezers showed decreases of more than 10% in CO2, and levels were even lower on day 90 (P < 0.05). Overall, analytes did not differ between the two −20 °C freezers through day 90.
Table 2.
Effects of storage in frost-free and nonfrost-free −20°C freezers on biochemical analytes in rat serum
| Mean (n = 10) percentage change from day 0 values | |||||
| Frost-free freezer | Nonfrost-free freezer | ||||
| Analyte | Mean ± SE, day 0 | day 30 | day 90 | day 30 | day 90 |
| Glucose, mg/dL | 153.3 ± 7.0 | 0.6 | 0.7 | 2.5 | 0.3 |
| Blood urea nitrogen, mg/dL | 21.25 ± 0.67 | 0.6 | 3.6 | 4.8 | (1.2) |
| Sodium, mmol/L | 145.8 ± 0.5 | (1.9) | (1.7) | (0.1) | (2.4) |
| Potassium, mmol/L | 5.61 ± 0.07 | 1.1 | 0.4 | 3.1 | (0.1) |
| Chloride, mmol/L | 97.8 ± 0.5 | 2.6 | (1.8) | 5.1 | (3.0) |
| Carbon dioxide, mmol/L | 32.5 ± 0.07 | (12.1)a | (21.5)a | (10.7)a | (18.3)a |
| Amylase, U/L | 1243.0 ± 37.6 | (1.0) | (2.6) | (2.5) | (4.7) |
| Lipase, U/L | 91.5 ± 2.7 | 0.4 | (0.7) | 2.8 | (0.3) |
| Calcium, mg/dL | 11.03 ± 0.08 | 1.2 | (1.7) | 2.8 | (0.8) |
| Phosphorus, mg/dL | 8.64 ± 0.15 | (1.6) | (3.3) | 0.6 | (0.6) |
| Total protein, g/dL | 6.35 ± 0.04 | 2.0 | 4.9 | 3.4 | 4.5 |
| Albumin, g/dL | 3.70 ± 0.04 | 1.7 | 3.6 | 5.5 | 1.1 |
| Aspartate aminotransaminase, U/L | 111.9 ± 3.5 | (1.8) | (2.8) | 1.1 | (4.0) |
| Alanine aminotransaminase, U/L | 92.6 ± 1.6 | (4.8) | (8.8) | (2.0) | (10.3) |
| Lactate dehydrogenase, U/L | 887.0 ± 54.2 | 0.3 | 1.8 | 2.5 | 0.6 |
| Creatine kinase, U/L | 178.1 ± 19.2 | (1.3) | (5.7) | (1.0) | (2.7) |
| Alkaline phosphatase, U/L | 208.9 ± 13.2 | (1.3) | (5.6) | (1.9) | (3.6) |
Values in parentheses indicate decreases from day 0 value.
Discussion
Storage of samples has been recognized as an important factor in human clinical pathology.-, Differences among the cited studies may be due to differences in sample handling before serum separation, storage temperatures, and test methodologies. In the veterinary literature, similar studies have been limited to canine and avian blood samples, which revealed interspecies differences in storage stability., Multispecies data from our laboratory (not shown) support this premise.
In the current study, refrigeration of rat serum for 7 d resulted in greater than a 10% decrease in creatine kinase activity. The activity of this enzyme in canine serum showed similar declines after storage at room temperature for 3 d. In addition, concentrations of CO2 in rat serum decreased by 9.4% over 7 d of refrigeration. This observation is consistent with findings from studies using human serum samples, for which loss of CO2 to the ambient atmosphere has been suggested as a cause for the differing concentrations.,
Freezing rat serum samples at −70 °C resulted in only modest changes in the analyte levels, with changes of less than 10% even after 360 d of storage. This finding is consistent with the human literature, which contains reports of analyte stability for 5 y. In addition, for many enzymes, storage in liquid nitrogen is superior to storage at −20 °C. Canine serum and plasma reportedly were more stable at −70 °C vs. -20oC over 240 d of storage, although both lactate dehydrogenase and amylase activity showed clinically significant changes (>10%).
Prolonged freezing of samples of rat serum in a frost-free −20 °C freezer led to many changes in analyte levels. These changes may be related to the overall higher temperature (relative to −70 °C). In the rat samples, alanine aminotransferase showed early significant decreases after 90 d. By day 360, greater than 50% decreases in alanine aminotransferase and creatine kinase were apparent, as were other less dramatic changes in lipase and alkaline phosphatase, for example. In the canine storage studies, alanine aminotransferase, alkaline phosphatase, and creatine kinase decreased, whereas amylase increased. Changes in enzyme activity are hypothesized to occur due to instability of enzyme isoforms. In addition, CO2 and calcium levels both showed changes of more than 10%. The CO2 changes appear consistent with those in the refrigerated samples. The observed increase in calcium after 360 d has not been reported previously.
To address differences between nonfrost-free and frost-free −20 °C freezers, we evaluated rat serum stored for 30 or 90 d. Notably, no significant differences were found between the freezers through the day 90 time point, with CO2 reaching a 18.3% decrease at day 90 in the nonfrost-free freezer compared with 21.5% in the frost-free freezer. Because most of the marked changes in analyte levels occurred between days 90 and 360, short-term (30 d) storage at −20 °C may be acceptable.
Throughout the multiple time points we assessed, the standard errors associated with the results were consistent among analytes. That is, no single sample showed marked instability. Because we obtained the samples from normal healthy rats, this result was expected. We presume that samples from clinically ill rats would behave similarly, but differences in enzyme isoforms and perhaps higher or lower starting values may be associated with particular sample instabilities. Further studies should be conducted to address this issue.
The present results show that, with the exception of creatine kinase activity, common biochemical analytes in rat serum are stable under refrigeration for 7 d. Whenever possible, prolonged sample storage should occur at −70 °C. If a −70 °C freezer is not available, −20 °C storage for as long as 90 d is acceptable for common analytes except CO2 and alanine aminotransferase. Retrospective studies and those requiring storage and batch analysis should accommodate these storage restrictions.
References
Abstract
The suitability of frozen serum after storage in primary sampling tubes with a gel separator for serological enzyme-linked immunosorbent assay testing (hepatitis B virus surface antigen [HBs Ag], anti-HBs Ag, anti-Toxoplasma gondii immunoglobulin G [IgG], anti-rubella virus IgG, anti-cytomegalovirus IgM, and anti-Epstein-Barr virus IgM) was evaluated for 375 samples. No difference was found among test results using fresh or stored frozen serum
Serum separator tubes were introduced into laboratories approximately 25 years ago and since have gained widespread acceptance due to the advantage of a barrier gel that facilitates rapid separation of serum from cells. Use of these tubes makes drawing blood easier, facilitates blood clotting and rapid separation of serum, reduces centrifugation time (they withstand higher centrifugation speed), and avoids transfer of serum to new tubes, contributing to improved quality of the preanalytical phase (, ).
Splice Serum Download Keeps Freezing Free
In general, serum for serology tests can be stored at 2 to 8°C for a few days before testing (1, 5, 10, ). Longer storage can be necessary, however, for confirmatory tests, quality control, seroepidemiological studies, demonstration of a significant increase in the titer of antibodies in paired acute and convalescent-phase sera, or other tests (6). For prolonged storage, the separated serum should be kept frozen in a new tube at −20°C or lower, avoiding repeated freeze-thaw cycles (5, 13). Here we report the results of an evaluation of the suitability of sera for serological testing when preserved frozen in serum gel separator primary sample collection tubes.
Samples.
We analyzed 375 sera received in our laboratory for serological studies. Blood (5 ml) was collected into a polyethylene terephthalate serum-gel-separator tube (Venojet II plastic vacuum tube; Terumo Europe, Leuven, Belgium). Within 4 h of the blood draw, tubes were centrifuged at 1,500 × g for 15 min, and initial testing was performed on the same day they were processed for storage.
After initial testing, gel separator tubes (containing the remaining serum, the gel, and the cell blood layer) were stored at −20°C, tightly capped with parafilm. In addition, from 140 out of the 375 samples, 0.5 ml of the serum was transferred to a polypropylene tube, which was also stored at −20°C. After 5 to 6 months' storage, the samples were thawed at room temperature and gently mixed, and serological analytes were determined again.
Serological tests.
The sera studied included positive and negative samples (Table (Table1)1) for hepatitis B surface antigen (HBs Ag), antibody to HBs Ag (anti-HBs Ag), anti-hepatitis C virus antibodies (anti-HCV Ag), anti-Toxoplasma gondii immunoglobulin G (IgG) antibodies (anti-Toxo IgG), anti-rubella virus IgG antibodies (anti-Rub IgG), anti-cytomegalovirus IgM antibodies (anti-CMV IgM), and anti-Epstein-Barr virus (VCA, EBNA, and EA antigens) IgM antibodies (anti-EBV IgM). All samples were tested using enzyme-linked immunosorbent assay (ELISA) microplate assays. Enzygnost tests (Dade-Bhering, Marburg, Germany) were used for all, with the exception of anti-HCV Ag, which was assayed using the Ortho HCV 3.0 ELISA test system (Ortho-Clinical Diagnostics, Inc., Raritan, N.J.). All testing was done in accordance with manufacturers' guidelines. Positive and negative controls were performed on each batch of tests. Serum samples (whether fresh or thawed) were directly handled using a 150 Genesis robotic sample processor (Tecan AG, Hombrechtikon, Switzerland), and further processing was performed in a Bhering ELISA processor III (Dade-Bhering).
TABLE 1.
Tests and number of sera used for evaluation of the suitability of frozen serum preserved in gel separator tubes for serological testing
| Serological testa | Sera studied qualitatively (step 1) | Sera studied qualitatively and quantitatively (step 2) | ||
|---|---|---|---|---|
| No. positive | No. negative | No. positive | No. negative | |
| HBs Ag | 18 | 25 | 10 | 10 |
| Anti-HBs Ag | 29 | 25 | 10 | 10 |
| Anti HCV Ag | 22 | 25 | 10 | 10 |
| Anti Toxo IgG | 11 | 11 | 10 | 10 |
| Anti-Rub IgG | 22 | 15 | 10 | 10 |
| Anti CMV IgM | 6 | 6 | 10 | 10 |
| Anti EBV IgM | 10 | 10 | 10 | 10 |
| Total | 118 | 117 | 70 | 70 |
The initial evaluation (step 1) of the suitability of frozen serum preserved in gel separator tubes for serological testing was carried out in 235 sera, comparing the qualitative results of the tests on the day of collection and after storage. Afterward (step 2), 140 sera, 10 positive and 10 negative for each serological test, were studied. We compared not only the qualitative results of the tests but also the quantitative results (absorbance readings) obtained from sera stored frozen in gel separator tubes and in polypropylene tubes. For each analyte, sera kept frozen in gel separator tubes and the fraction kept frozen in polypropylene tubes (in total, 40 samples) were thawed and analyzed in a run and in a single microplate to avoid interassay variability.
There was total agreement between all qualitative results for the 375 sera (Table (Table1,1, steps 1 and 2) that were tested on the day of collection and after being stored frozen in gel separator tubes. No misclassification was noticed in any serological test using sera stored frozen either in polypropylene tubes or in gel separator tubes. No significant difference (paired t test) was found among the absorbance readings obtained with the 140 sera preserved frozen in propylene tubes and in gel separator tubes (Table (Table2).2).
TABLE 2.
Comparison of absorbance values of 140 frozen sera for seven serological ELISA tests after five months' storage in gel separator tubes and in polypropylene tubesa
| Test | Results for Positive sera (n = 70, 10 for each test) | Results for negative sera (n = 70, 10 for each test) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Positive cutoffb | Absorbance range | Δ Absorbancec | Pd value | Negative cutoffe | Absorbance range | Δ Absorbance | P value | |||
| Gel separator tube | Polypropylene tube | Gel separator tube | Polypropylene tube | |||||||
| HBs Ag | 0.088 | 0.532-3.847 | 0.430-3.740 | 0.029 ± 0.3 | 0.75 | 0.088 | 0.025-0.041 | 0.024-0.042 | 0.001 ± 0.07 | >0.9 |
| Anti-HBs Ag | 0.125 | 0.342-3.413 | 0.401-3.433 | 0.019 ± 0.1 | 0.85 | 0.125 | 0.038-0.103 | 0.040-0.102 | 0.003 ± 0.09 | >0.9 |
| Anti HCV Ag | 0.379 | 0.693-3.734 | 0.758-3.891 | 0.029 ± 0.3 | 0.75 | 0.379 | 0.022-0.043 | 0.026-0.045 | 0.001 ± 0.08 | >0.9 |
| Anti Toxo IgG | 0.127 | 0.410-1.815 | 0.476-1.760 | 0.067 ± 0.3 | 0.50 | 0.047 | 0.020-0.035 | 0.014-0.039 | 0.002 ± 0.08 | >0.9 |
| Anti-Rub IgG | 0.200 | 0.658-2.118 | 0.551-2.239 | 0.050 ± 0.3 | 0.65 | 0.100 | 0.004-0.032 | 0.006-0.033 | 0.003 ± 0.07 | >0.9 |
| Anti CMV IgM | 0.200 | 0.241-1.034 | 0.212-1.260 | 0.007 ± 0.3 | >0.9 | 0.100 | 0.003-0.072 | 0.002-0.084 | 0.004 ± 0.12 | >0.9 |
| Anti EBV IgM | 0.170 | 0.325-0.725 | 0.346-0.817 | 0.030 ± 0.3 | 0.7 | 0.100 | 0.001-0.064 | 0.003-0.070 | 0.001 ± 0.09 | >0.9 |
After thawing, eight (2.1%) out of the 375 sera kept frozen in gel separator tubes showed a light red color, indicating passage of hemoglobin through the gel. Of these hemolyzed samples, two were positive for HBs Ag, one was positive for anti-HCV Ag, one was positive for anti-Toxo IgG, two were negative for Anti-Rub IgG, one was negative for HBs Ag, and one was negative for anti-HBs Ag. Qualitative results from all eight hemolyzed sera (gel separator tubes) showed no misclassification when they were compared with nonhemolyzed sera, either fresh or preserved frozen in a polypropylene tube. Three out of these eight sera (one negative for HBs Ag, one negative for anti-HBs Ag, and one positive for anti-Toxo IgG) were studied quantitatively (step 2). No significant change in absorbance values in the ELISA tests was noticed between the frozen serum stored either in the gel separator tube (slightly hemolyzed) or in the polypropylene tube. The difference between the absorbance readings for each of these sera pairs was always within the standard deviation for the differences of absorbances in the test.
In general, gel separator tubes are suitable for collection and short-term storage of blood for commonly ordered laboratory tests (). But due to absorption of drugs by the gel, a significant reduction in the concentration of therapeutic drugs when blood is kept in these tubes has been reported (, ). We have not, however, found significant differences between the Ig concentrations (absorbance readings in the ELISA tests) in serum samples kept frozen in polypropylene tubes and those in samples kept in gel separator tubes.
A point of concern when dealing with clinical chemistry analytes is hemolysis. If this is present it can cause spurious tests results, and estimation of some analytes (e.g., hydroxybutyrate dehydrogenase, aspartate transaminase, creatine, bicarbonate, and potassium) are not valid (, , ). It has also been reported that hemolyzed serum may not be suitable for serological testing (5). In this study results were not affected because of passage of some hemoglobin through the gel. Nevertheless, since this happened in only a few sera, further work is necessary before any general conclusion can be drawn on this point.
The purpose of quality control is to prevent as many errors as possible and to detect those which do occur, but according to Murphy's Law, if anything can go wrong, it will (13). Handling of sera for storage not only is prone to technical and clerical errors (labeling of tubes and keeping the inventory) but also can lead to serious biological risks, since it involves handling potentially highly infectious material (hepatitis B and C virus, human immunodeficiency virus, and other potential pathogens). The finding that serum samples for ELISA tests can be stored at −20°C in primary tubes with a serum gel separator can avoid potential errors in sample identification and can decrease the workload associated with serum storage. This can also minimize the risks of biohazard associated with the transfer of sera to new tubes. In conclusion, we have found that stowing frozen centrifuged blood in the primary collection tube with a gel separator barrier could be an alternative to the transfer of serum to new tubes for storage. Further studies will be necessary, however, to prove that this approach is generally valid for serological tests.